BIOFIRE® Blood Culture Identification 2 (BCID2) Panel
1 Test. 43 Targets. ~1 Hour.
The BIOFIRE BCID2 Panel tests for 43 targets associated with bloodstream infections, including Gram-negative bacteria, Gram-positive bacteria, yeast, and 10 antimicrobial resistance genes – all with one test and with results available in about an hour from positive blood culture.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
- Overview
- Panel Menu
- Services & Support
- Specs & Resources
Overview
The BIOFIRE® Blood Culture Identification 2 (BCID2) Panel tests a single positive blood culture sample to simultaneously provide results for multiple organisms and organism groups that cause bloodstream infections, as well as genetic markers associated with antimicrobial resistance. It is designed for use on the BIOFIRE® FILMARRAY® TORCH System, an FDA-cleared, CE-IVD, and TGA certified multiplex PCR system that integrates sample preparation, amplification, detection, and analysis.
Fast, Accurate, Comprehensive
Obtaining rapid blood culture organism identification, combined with the local antibiogram, allows for the timely adjustment of a broad-spectrum empiric antimicrobial therapy to a narrow, targeted patient treatment.1* The potential benefits of fast and comprehensive testing of bloodstream infections may include guided appropriate antibiotic therapy, decreased hospital length of stay2**, reduced hospital costs, and reduced patient morbidities and mortality.
BIOFIRE BCID2 Panel can provide the essential combination of speed, accuracy, and comprehensiveness to enable a rapid, definitive identification of a pathogen directly from positive blood cultures. With just one test, you can identify pathogens in more than 9 out of 10 positive blood cultures in about 1 hour with only 2 minutes of hands-on time.
- Simple: 2 minutes hands-on time
- Fast: Turnaround time of about 1 hour
- Comprehensive: Simultaneously tests for 43 targets and identifies pathogens in more than 9 out of 10 positive blood cultures. The BIOFIRE BCID2 Panel detects 10 antimicrobial resistance genes and emerging pathogens like C. auris and S. maltophilia.
- Accurate: The average positive agreement rate (or sensitivity) across all pathogens on the BIOFIRE BCID2 Panel is 99.0%, and the negative agreement rate (or specificity) is 99.8%3
Fighting Sepsis
Enhanced Antimicrobial Stewardship
The Infectious Diseases Society of America (IDSA) guidelines recommend the addition of rapid diagnostic testing to an effective Antimicrobial Stewardship Program.7 A study at the Mayo Clinic shows that pairing the Panel with antimicrobial stewardship provides the greatest benefit.*1
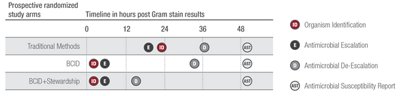
Adapted from Banerjee R, et al. Clinical Infectious Diseases 2015;61(7):1
Shorten Time to Optimal Therapy
Reduce Hospital Costs
Faster organism identification and reduced time to optimal therapy can also lead to better economic outcomes. The identification of coagulase-negative staphylococci contaminants resulted in shorter post-culture length of stay and saved roughly $30,000 per 100 patients tested (obtained in a pre/post interventional study on an adult population).9‡
*Data obtained with the previous version of the BIOFIRE BCID2 Panel.
**Data obtained with the previous version of the BIOFIRE BCID2 Panel in a pediatric population.
†Data obtained with the previous version of the BIOFIRE BCID2 Panel in a pediatric population and compared to Standard of Care (SOC).
‡Data obtained with the previous version of the BIOFIRE BCID2 Panel in a pre/post interventional study on an adult population.
1. Banerjee R, et al. Randomized Trial of Rapid Multiplex Polymerase Chain Reaction-Based Blood Culture Identification and Susceptibility Testing. Clin Infect Dis. 2015;61:1071-80.
2. Ray et al. Pediatr Infect Dis J 2016;35:e134-138
3. The stated performance is the aggregate of the prospective data from the clinical study.
4. Sepsis Alliance: Sepsis Fact Sheet 2018. https://www.sepsis.org/wp-content/uploads/2017/05/Sepsis-Fact-Sheet-2018.
5. Rhodes et al., Intensive Care Med. 2017 Mar;43(3):304-377
6. Zhang D, et al. Critical Care Medicine 2015;43(10):2133-2140
7. Barlam T, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Infectious Diseases Society of America. 2016;62(15 May).
8. Messacar K, et al. Clinical Impact and Provider Acceptability of Real-Time Antimicrobial Stewardship Decision Support for Rapid Diagnostics in Children With Positive Blood Culture Results. J Ped Infect Dis Soc. 2017;6(3):267-274.
9. Pardo J, et al. Clinical and Economic Impact of Antimicrobial Stewardship Interventions with the FilmArray® Blood Culture Identification Panel. Diagn Microbiol Infect Dis. 2016;84(2):159–164.
Services & Support
Customer Support: bioMérieux offers the best possible support with dedicated BIOFIRE® technical experts.
Application Specialists: bioMérieux has dedicated support teams who are available to help set up equipment and troubleshoot any issues.
For assistance: please contact our customer technical support team at biofiresupport@biomerieux.com
Specs & Resources
Technical Panel Specifications
| Sample Handling | Performance Parameters |
|---|---|
| Sample Type: Positive Blood Culture Media | Hands-on Time: Approx. 2 minutes |
| Sample Volume: 0.2 mL | Run Time: ~ 1 Hour |
| Product Name | Part Number | Quantity |
|---|---|---|
| BIOFIRE® BCID2 Panel Reagent Kit | RFIT-ASY-0147 | 30 Pouches |