BIOFIRE® FILMARRAY® Pneumonia (PN) & Pneumonia plus (PNplus) Panels
1 Test. Up to 34 Targets. ~1 Hour.
The BIOFIRE PN and PNplus Panels target a comprehensive menu of bacteria and viruses associated with pneumonia and other lower respiratory tract infections, as well as seven antimicrobial resistance markers.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
- BIOFIRE FILMARRAY PN Panel
- Overview
- Panels Menu
- Services & Support
- Specs & Resources
Overview
Syndromic Infectious Disease Testing for Pneumonia
The overlapping symptoms of pneumonia patients make it hard to pinpoint the causative pathogen. As such, patients are often prescribed antimicrobials that may not be appropriate or necessary. The BIOFIRE® FILMARRAY® Pneumonia (PN) and Pneumonia plus (PNplus) Panels deliver fast, accurate pathogen identification in about one hour, which may allow physicians to initiate appropriate therapy more promptly for patients, reducing unnecessary antibiotic use and improving overall care.1,2,3
The BIOFIRE PN and PNplus Panels identify over 30 clinically relevant targets from sputum-like (sputum or endotracheal aspirate) or bronchoalveolar lavage (BAL)-like (BAL or mini-BAL) specimens. For 15 bacteria, the panels provide semi-quantitative results, which may help determine whether an organism is a colonizer or a pathogen. The fast and comprehensive panels are run on the BIOFIRE®FILMARRAY® TORCH System, which integrates sample preparation, amplification, detection, and analysis.
- Simple: 2 minutes hands-on time
- Fast: Turnaround time of about 1 hour
- Comprehensive:
- BIOFIRE PN Panel: 33 targets (18 bacteria, 8 viruses, 7 antimicrobial resistance genes)
- BIOFIRE PNplus Panel: 34 targets (18 bacteria, 9 viruses, 7 antimicrobial resistance genes)
- Accurate:
- BAL-like: 96.2% sensitivity and 98.3% specificity (PNplus: 98.4%)1
- Sputum-like: 96.3% sensitivity and 97.2% specificity (PNplus: 97.3%)1
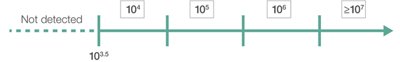
Semi-quantitative Results for 15 Common Colonizers
Values Reported by the BIOFIRE PN and PNplus Panels
Superior Clinical Outcomes
Fast and accurate identification of the causative agent of both community- and healthcare-associated respiratory infections can help improve patient management by informing timely and effective antibiotic or antiviral therapy. A quick diagnosis can assist with directing appropriate infection control practices thereby aiding in the prevention of secondary spread of infection, shorten hospital stays, reduce ancillary testing, and reduce overall health care costs.
References
1. Data on File at BioFire Diagnostics.
2. Buchan BW, Windham S, Balada-Llasat JM, Leber A, Harrington A, Relich R, et al. Practical Comparison of the BioFire FilmArray Pneumonia Panel to Routine Diagnostic Methods and Potential Impact on Antimicrobial Stewardship in Adult Hospitalized Patients with Lower Respiratory Tract Infections. J Clin Microbiol. 2020;58(7).
3. Poole S, Tanner AR, Naidu VV, Borca F, Phan H, Saeed K, et al. Molecular point-of-care testing for lower respiratory tract pathogens improves safe antibiotic de-escalation in patients with pneumonia in the ICU: results of a randomised controlled trial. The Journal of infection. 2022.
Services & Support
Customer Support: bioMérieux offers the best possible support with dedicated BIOFIRE® technical experts.
Application Specialists: bioMérieux offers dedicated support teams who are available to help set up equipment and troubleshoot any issues.
For assistance: please contact our customer technical support team at biofiresupport@biomerieux.com
Specs & Resources
Technical Panel Specifications
| Sample Handling | Performance Parameters |
|---|---|
Sample Type: Sputum-like: Induced and expectorated Sputum; Endotracheal aspirates. BAL-like: BAL and mini-BAL | Hands-on time: Approx. 2 minutes |
Sample Volume: 0.2 mL collected with a flocked swab | Run time: ~1 Hour |
| Product Name | Part Number | Quantity |
|---|---|---|
| BIOFIRE® PN Panel Reagent Kit | RFIT-ASY-0144 | 30-Pouches |
| BIOFIRE® PNplus Panel Reagent Kit | RFIT-ASY-0143 | 30-Pouches |
%20Panel%20Information%20Sheet%20Thumbnail?qlt=85&ts=1701687117999&dpr=off)
%20Panel%20Information%20Sheet%20Thumbnail?qlt=85&ts=1701687123425&dpr=off)
%20Panel%20Brochure%20Thumbnail?qlt=85&ts=1701687128466&dpr=off)
%20Panel%20Brochure%20Thumbnail?qlt=85&ts=1701687133661&dpr=off)