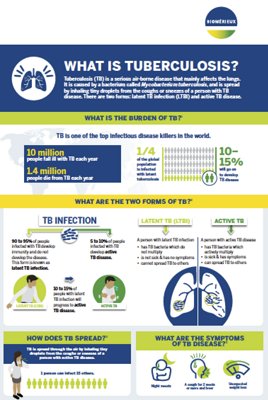
What is Tuberculosis?
Tuberculosis is a communicable disease caused by various strains of mycobacteria, most frequently Mycobacterium tuberculosis. The disease usually affects the lungs (pulmonary tuberculosis), but it can manifest anywhere outside the lungs as well (extrapulmonary tuberculosis). Tuberculosis spreads through person-to-person transmission, such as when individuals with pulmonary tuberculosis release bacteria through coughing. There are two main forms of TB—latent TB infection (LTBI), and active TB. People with LTBI are asymptomatic and cannot infect others; however, when those with LTBI develop active TB, they can then infect other people.
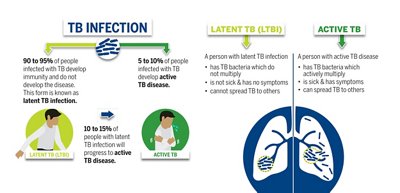
*Retrieved from https://www.cdc.gov/tb/publications/infographic/default.htm
What is the Healthcare Burden of Tuberculosis?
Tuberculosis remains a major global public health problem and one of the leading causes of death worldwide, with nearly 4,000 lives lost every day.1,2 Over 10 million new cases are recorded each year. Additionally, a quarter of the global population is infected with latent tuberculosis, making LTBI a large reservoir for TB, which is why managing LBTI is critical for reducing and eliminating TB worldwide.
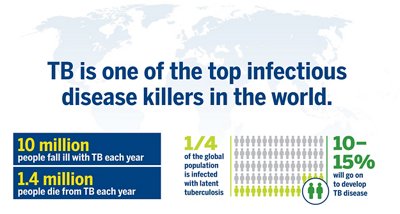
**Retrieved from https://www.who.int/tb/WTBD2019infographics.pdf
Treatment for TB involves taking a combination of drugs for several months, which is costly and frequently causes side effects. As a result, patients can find it difficult to adhere to treatment regimens. Unfortunately, incomplete or incorrectly administered treatment can lead to antimicrobial resistance. Multidrug resistant and extensively drug-resistant TB variants are more difficult and costly to treat, and they have higher mortality rates.
Diagnostic Challenges with Tuberculosis
With prompt diagnosis and appropriate antibiotic treatment, most people with TB can be cured. However, diagnosis of TB is often delayed, due to multiple factors including a lack of knowledge or awareness of TB, non-specific symptoms, and inadequate access to healthcare. Additionally, diagnosing active TB and latent TB involve different approaches.
Methods for diagnosing active TB include bacteriological confirmation and clinical diagnosis. Bacteriological confirmation, including traditional microbial culture, lateral flow urine lipoarabinomannan (LF-LAM) assays, sputum smear microscopy, or rapid molecular tests, is a necessary step for identifying TB that is resistant to antimicrobials.
Methods for diagnosing latent TB include the tuberculin skin test (TST) and interferon-gamma release assays (IGRA). Adequate diagnosis and treatment of latent tuberculosis infection is one of the critical components of the World Health Organization’s End TB Strategy, essential to prevent the development of active tuberculosis disease and stop the spread of tuberculosis.
Our Solutions for Diagnosing Tuberculosis
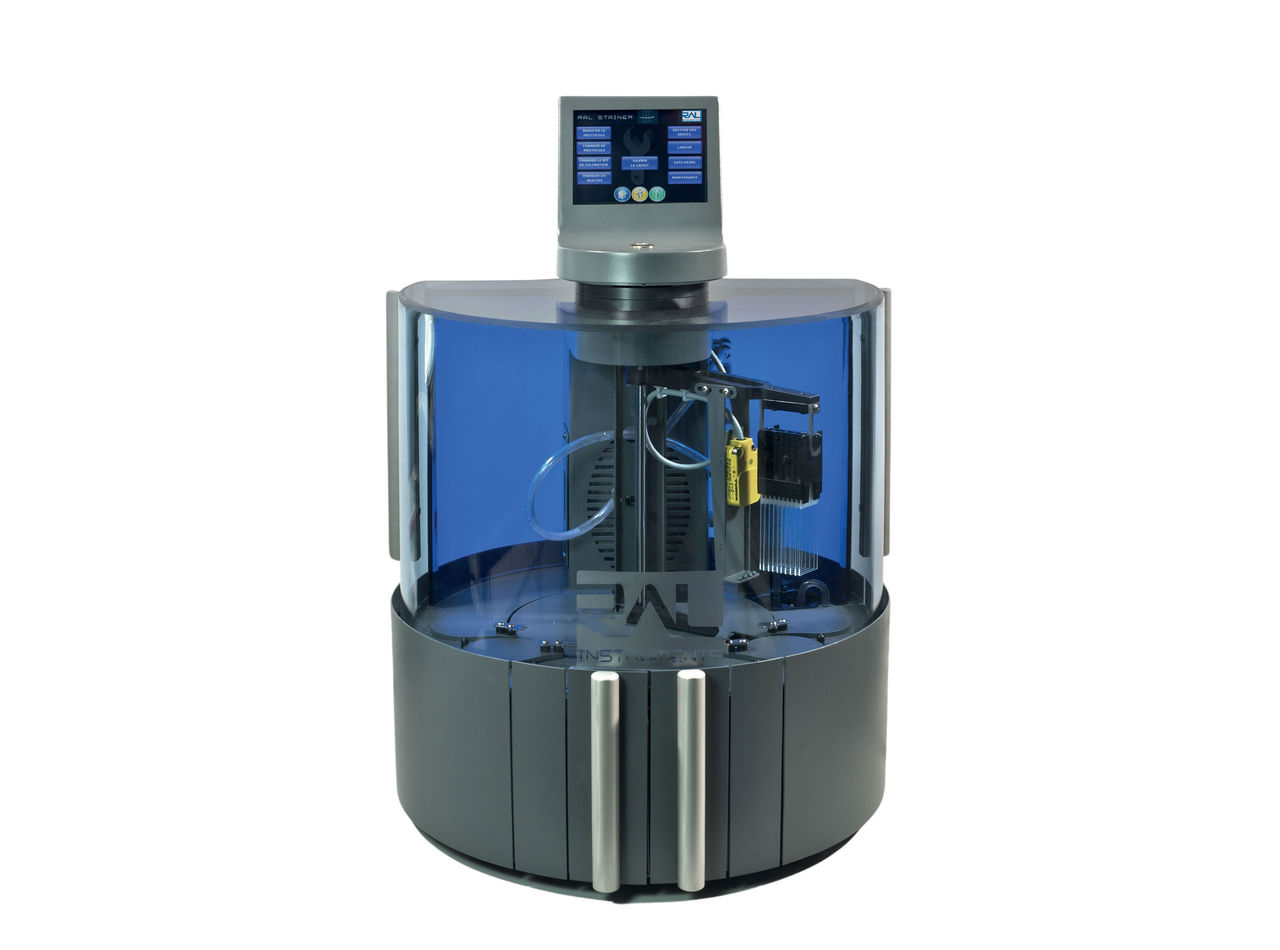
For active tuberculosis infections, our RAL STAINER helps standardize AFB (Acid-fast bacteria) staining for sputum smear microscopy. WHO guidelines recommend fluorescence as the reference method for staining and notes that Thiazine Red makes reading easier. The RAL STAINER and its reagents help ensure your lab meets these guidelines.
Our VITEK® MS and VITEK® MS PRIME database includes Mycobacterium tuberculosis complex and is consistently expanding to add new emerging pathogens and clinically relevant species.
For latent tuberculosis infection, our interferon gamma production detection test, VIDAS® TB-IGRA, is simple, effective, and reliable. Its high sensitivity and specificity provide reliable results and an improved ability to detect Mycobacterium tuberculosis in infected individuals.
Tuberculosis - Our Diagnostic Offer
bioMérieux offers solutions for diagnosing both active and latent tuberculosis.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
-
VIDAS® TB-IGRA
Reliable and fully automated solution for Tuberculosis (TB) infection detection
Available on the VIDAS® 3 immunoanalyzer, VIDAS® TB-IGRA is a whole-blood test for the diagnosis of Mycobacterium tuberculosis infection.
-
RAL® STAINER
Secures Your AFB Staining and Eases Slide Reading
Easier and faster reading and no daily maintenance with RAL® STAINER. This closed fully automated bath system offers a unique fixative solution that prevents any cross-contamination.
-
BACT/ALERT® Culture Media Bottles
Innovative & Optimized Culture Media
BACT/ALERT® culture media offers a comprehensive range of media bottles to ensure the recovery of a wide variety of microorganisms including bacteria, mycobacteria, and fungi – including our next generation Fastidious Antimicrobial Neutralization Plus media (FAN® PLUS) offering optimized time to detection and recovery.
-
VITEK® MS
Microbial Identification in Minutes
VITEK® MS provides rapid and robust identification of microorganisms delivering clinicians with actionable results to support informed treatment decisions.
-
VITEK® MS PRIME
New-Generation Mass Spectrometry Microbial Identification System
VITEK® MS PRIME combines microbiology expertise with innovation taking mass spectrometry to the next level by maximizing the impact of daily laboratory workflow for better patient care.
Useful Resources on Tuberculosis
Contact Us for Information on Tuberculosis Solutions
References
1. Global Tuberculosis Programme. World Health Organization. Retrieved December 13, 2023, from https://www.who.int/teams/global-tuberculosis-programme/tb-reports
2. Tuberculosis. European Centre for Disease Prevention and Control. Retrieved December 13, 2023, from https://www.ecdc.europa.eu/en/tuberculosis